


Brief Comments on Revoking the Trademark “NATULAN” (No. 768117) for non-use of Three Years
Recently, in the case of revoking Lidi Ante Biological Science Co., Ltd., trademark “NATULAN” with No. 768117 (hereinafter referred to as “the registered trademark”) on Class 5 “pharmaceutic preparation”, China National Intellectual Property Administration hold that Lidi Ante Biological Science Co., Ltd.’s ground for the non-use of the registered trademark within the designated period is justified, the registration of the trademark should be retained.
The Basic Facts
The trademark “NATULAN” with No. 768117 has been registered on 28th September 1995, currently, it has been owned by Lidi Ante Biological Science Co., Ltd. the registration is under Class 5, on the goods “pharmaceutic preparation”. According to Article 49 of Chinese Trademark Law, a company has filed the request for invalidating the registered trademark based on the ground of non-use of the trademark for concessive three years without justification. The registrant has defended within the statutory time limit, stated the right reason for non-use of the registered trademark between 10th June 2017 and 9th June 2020, furthermore, actively prepared for the use of the registered trademark. After the review, China National Intellectual Property Administration held that the registrant has the right reason for non-use of registered trademark, as a result, the registration of the trademark shall be sustained.
The Rule of China National Intellectual Property Administration
Lidi Ante Biological Science Co., Ltd.’s trademark “NATULAN” with registered No. 768117 has the justification for non-use on the goods “pharmaceutic preparation”. As per Article 49 of Chinese Trademark Law, as well as Article 66 and Article 67 of Implementation Regulations on Chinese Trademark Law, the trademark “NATULAN” with registered No. 768117 should be sustained.
Analysis on the Case
1.The registered trademark has not put into use practically because of the registrant’s right reason that the registrant shall not be liable.
As per Article 67 of Implementation Regulations on Chinese Trademark Law: followings are justifications stipulated by Article 49 of Chinese Trademark Law:
(1)Force Majeure
(2)The government’s policy-oriented restrictions
(3)Bankruptcy
(4)Other right reasons that the registrant shall not be liable.
As per Article 26 of Regulations of Several Questions on the Supreme People’s Court Reviewing Administrative Cases regarding the Confirmation and Authorization of Trademarks (Fa Shi (2017) No.2): …
“If the trademark owner has the real intention to use the trademark, also, has necessary preparation for the practical use, however, the registered trademark has not put into practical use due to objective reason, the People’s court could hold that the owner has the right reason.
As per the Article 24 of The Drug Administration Law of the People’s Republic of China, medicines listed in China should be approved by the drug supervision department of the State Council, also, should obtain the medicine registration certificate.
In this case, Sigma Tuo Pharmaceutical Industry Combination Joint-Stock Company, the original registrant of the registered trademark, has entrusted Zhaoke Pharmacy (Hefei) Co., Ltd., to conduct clinical test in China Mainlan between 29th December 2016 and 20th February 2019. Zhaoke Pharmacy (Hebei) Co., Ltd. is accountable for making overall arrangement for Natulan medicine’s clinical in China mainland and official application work. As per the research report of the clinical test (clinical research approval No. 2015L00386), the medicine with the registered trademark has been put into clinical test and research in China between 29th December 2016 and 20th February 2019.
After the transfer of the registered trademark by the registrant and the completion of the research of clinical test for the medicine with the registered trademark, the registrant and the trustee, Zhaoke Pharmacy (Hefei) Co., Ltd. actively applied to the State Drug Administration for the imported medicine to be listed. The State Drug Administration has already issued the notice of acceptance on 28th April 2020, the acceptance No. was JXHS2000053 GUO, currently, the medicine still in the process of approval period for the list. In view of the special feature of the medicine industry, the medicine to be listed in China, should be approved by the Drug Administration of State Council, and then put into production and sale after obtaining the medicine registration certificate and passing the inspection.
The period of clinical test and approval of application for the list just coincided with the designated using period, i.e. between 10th June 2017 and 9th June 2020.
In this case, the registrant has the statutory justification for non-use of the registered trademark within statutory time limit, which should not be attributed to the registrant, pertain to the situation, i.e, “the trademark should keep the registration”, which is stipulated by Article 67 in Implementation Regulations on Trademark Law, and Article 26 in “Regulations on Several Several Questions on the Supreme People’s Court Reviewing Administrative Cases regarding the Confirmation and Authorization of Trademarks. As a result, the registration of the registered trademark should be maintained according to the law.
2.In the priorly similar cases, the court showed that if the trademark owner has the intention for the use of the registered trademark, moreover, the owner actually made necessary preparation, has the right reasons for the non-use of the registered trademark, the registration of the registered trademark should be maintained.
Court’s relevant cases are as follows:
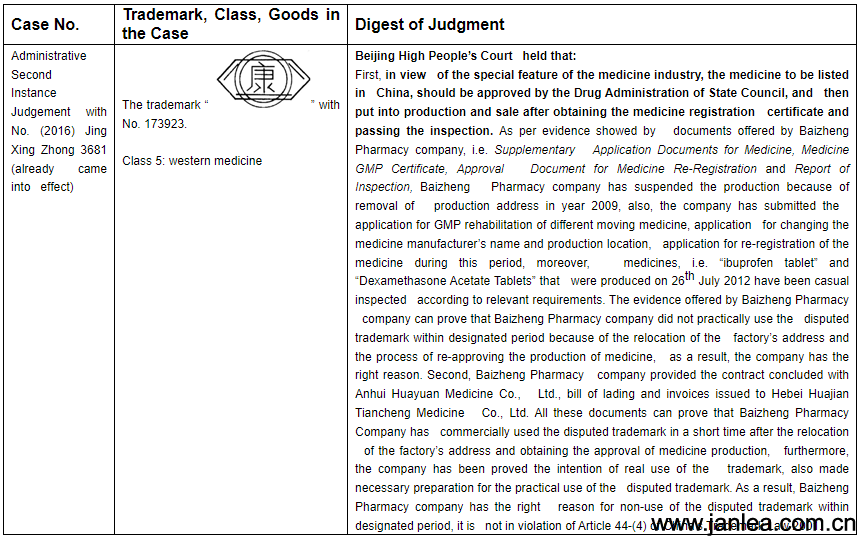
3. Advice on Burden of Proof
In this case, we have sorted out the basic facts of the whole case, based on the fact that the medicine with the registered trademark has completed the clinical test and applied to the competent authority for the list, we advised to offer t the medicine’s clinical test report, the imported medicine’s application form for the registration that was authorized by the client to China agent to submit to the State Drug Administration, the notice of acceptance issued by the State Drug Administration and other evidence in written. Based on such kind of evidence, furthermore, we have provided the State Intellectual Property Office with search result of progress of medicine registration, which was captured from the State Drug Administration, Chinese press reports regarding Natulan medicine has been reported for the approval. So far, the registrant’s real intention for using the registered trademark could be proved all filed evidence, also, the active implementation of relevant procedures could be proved, approval documents of producing medicine have been filed to the State Drug Administration, as a result, there were right reasons for the non-use of the registered trademark within time limit. It can be seen that, for medicine companies, they should active use trademarks, in case of similar revocation in this case, they should provide evidential materials and vouchers for the preparation of actual use of registered trademarks when they do not put it into actual use.
 业务领域:
业务领域: 此案件代理人
此案件代理人
